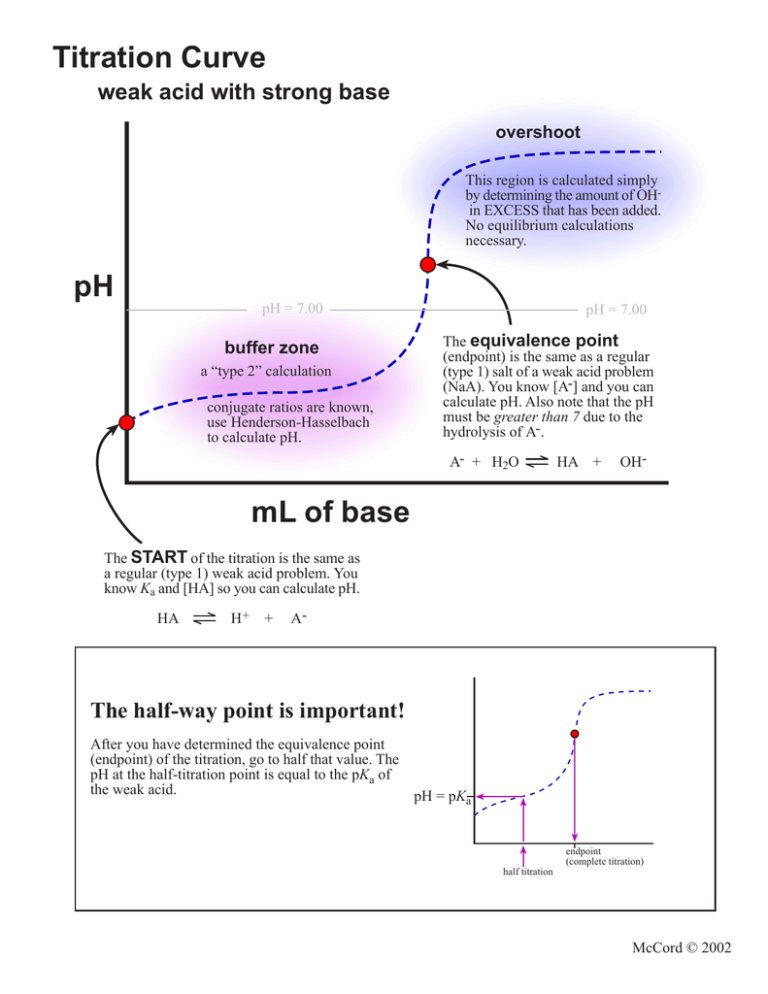
Titration Curve Important Points . titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the independent. The way you normally carry out a titration involves adding the acid to the alkali. a titration curve is a graphical representation of the ph of a solution during a titration. one particularly important point in a titration curve is the equivalence point. updated on june 26, 2019. titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. Titration is a technique used in analytical chemistry to determine the concentration of an unknown acid or base. The end point of a titration is the point at which an. a summary of the important curves.
from studylib.net
one particularly important point in a titration curve is the equivalence point. a titration curve is a graphical representation of the ph of a solution during a titration. a summary of the important curves. titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the independent. The way you normally carry out a titration involves adding the acid to the alkali. Titration is a technique used in analytical chemistry to determine the concentration of an unknown acid or base. The end point of a titration is the point at which an. updated on june 26, 2019.
Titration Curve weak acid with strong base overshoot
Titration Curve Important Points a titration curve is a graphical representation of the ph of a solution during a titration. updated on june 26, 2019. titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. The end point of a titration is the point at which an. The way you normally carry out a titration involves adding the acid to the alkali. one particularly important point in a titration curve is the equivalence point. titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the independent. Titration is a technique used in analytical chemistry to determine the concentration of an unknown acid or base. a titration curve is a graphical representation of the ph of a solution during a titration. a summary of the important curves.
From mavink.com
Strong Acid And Base Titration Curve Titration Curve Important Points a titration curve is a graphical representation of the ph of a solution during a titration. Titration is a technique used in analytical chemistry to determine the concentration of an unknown acid or base. titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the independent. The way you normally. Titration Curve Important Points.
From chem.libretexts.org
9.4 Redox Titrations Chemistry LibreTexts Titration Curve Important Points The way you normally carry out a titration involves adding the acid to the alkali. one particularly important point in a titration curve is the equivalence point. The end point of a titration is the point at which an. updated on june 26, 2019. Titration is a technique used in analytical chemistry to determine the concentration of an. Titration Curve Important Points.
From ambitiousmares.blogspot.com
30 Label The Important Points Of The Titration Curve Below. Labels Design Ideas 2020 Titration Curve Important Points one particularly important point in a titration curve is the equivalence point. The way you normally carry out a titration involves adding the acid to the alkali. a summary of the important curves. updated on june 26, 2019. titration curves show how the ph of an acidic or basic solution changes as a basic or acidic. Titration Curve Important Points.
From www.youtube.com
Modelling an EDTA Titration Curve YouTube Titration Curve Important Points Titration is a technique used in analytical chemistry to determine the concentration of an unknown acid or base. a summary of the important curves. a titration curve is a graphical representation of the ph of a solution during a titration. The way you normally carry out a titration involves adding the acid to the alkali. The end point. Titration Curve Important Points.
From dxolbfltl.blob.core.windows.net
Halfway Point For Titration Curve at Mildred Cruz blog Titration Curve Important Points Titration is a technique used in analytical chemistry to determine the concentration of an unknown acid or base. a titration curve is a graphical representation of the ph of a solution during a titration. titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the independent. one particularly important. Titration Curve Important Points.
From kwokthechemteacher.blogspot.com
KWOK The Chem Teacher ionic equilibrium titration curves Titration Curve Important Points a summary of the important curves. titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the independent. The way you normally carry out a titration involves adding the acid to the alkali. titration curves show how the ph of an acidic or basic solution changes as a basic. Titration Curve Important Points.
From ar.inspiredpencil.com
Titration Curve Labeled Titration Curve Important Points a summary of the important curves. The way you normally carry out a titration involves adding the acid to the alkali. titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. Titration is a technique used in analytical chemistry to determine the concentration of an. Titration Curve Important Points.
From studylib.net
Titration Curve weak acid with strong base overshoot Titration Curve Important Points The end point of a titration is the point at which an. titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. one particularly important point in a titration curve is the equivalence point. The way you normally carry out a titration involves adding the. Titration Curve Important Points.
From www.chegg.com
Solved Question 1 At which point in the titration curve Titration Curve Important Points a titration curve is a graphical representation of the ph of a solution during a titration. a summary of the important curves. The end point of a titration is the point at which an. The way you normally carry out a titration involves adding the acid to the alkali. titration curves show how the ph of an. Titration Curve Important Points.
From mainpackage9.gitlab.io
Cool Titration Curve In Excel Broken Axis Titration Curve Important Points one particularly important point in a titration curve is the equivalence point. a summary of the important curves. a titration curve is a graphical representation of the ph of a solution during a titration. titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the independent. Titration is. Titration Curve Important Points.
From hicensvanderkruijs.blogspot.com
The Graph Shows The Titration Curves Of A 1M Solution / Consider The Titration Curve Given For Titration Curve Important Points a titration curve is a graphical representation of the ph of a solution during a titration. The end point of a titration is the point at which an. Titration is a technique used in analytical chemistry to determine the concentration of an unknown acid or base. titrations are often recorded on graphs called titration curves, which generally contain. Titration Curve Important Points.
From chem.libretexts.org
9.4 Redox Titrations Chemistry LibreTexts Titration Curve Important Points a summary of the important curves. The way you normally carry out a titration involves adding the acid to the alkali. updated on june 26, 2019. a titration curve is a graphical representation of the ph of a solution during a titration. titrations are often recorded on graphs called titration curves, which generally contain the volume. Titration Curve Important Points.
From crunchchemistry.co.uk
How to explain the shape of a titration curve Crunch Chemistry Titration Curve Important Points updated on june 26, 2019. The way you normally carry out a titration involves adding the acid to the alkali. a titration curve is a graphical representation of the ph of a solution during a titration. one particularly important point in a titration curve is the equivalence point. a summary of the important curves. titration. Titration Curve Important Points.
From dquxjfqneco.blob.core.windows.net
Titration Curve In Biochemistry at Cynthia Devitt blog Titration Curve Important Points The end point of a titration is the point at which an. titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. a summary of the important curves. updated on june 26, 2019. The way you normally carry out a titration involves adding the. Titration Curve Important Points.
From www.writework.com
Titration of amino acids WriteWork Titration Curve Important Points titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. one particularly important point in a titration curve is the equivalence point. a summary of the important curves. The way you normally carry out a titration involves adding the acid to the alkali. . Titration Curve Important Points.
From www.chemistrystudent.com
Finding Ka using a Titration Curve (A2level) ChemistryStudent Titration Curve Important Points The way you normally carry out a titration involves adding the acid to the alkali. Titration is a technique used in analytical chemistry to determine the concentration of an unknown acid or base. a titration curve is a graphical representation of the ph of a solution during a titration. updated on june 26, 2019. titration curves show. Titration Curve Important Points.
From www.expii.com
What Is a Titration Curve? — Overview & Parts Expii Titration Curve Important Points a titration curve is a graphical representation of the ph of a solution during a titration. The way you normally carry out a titration involves adding the acid to the alkali. one particularly important point in a titration curve is the equivalence point. The end point of a titration is the point at which an. updated on. Titration Curve Important Points.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent Titration Curve Important Points titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the independent. The end point of a titration is the point at which an. a titration curve is a graphical representation of the ph of a solution during a titration. Titration is a technique used in analytical chemistry to determine. Titration Curve Important Points.